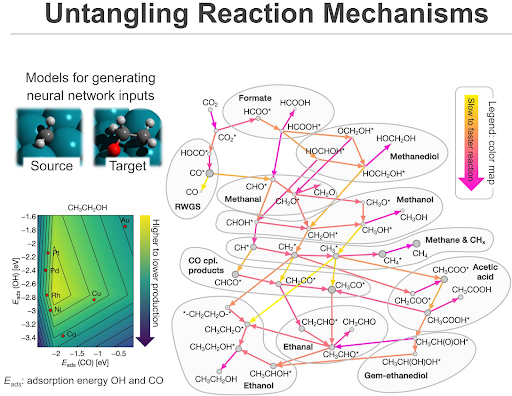
This project focuses on tackling one of the key challenges in carbon utilisation — converting CO₂ into value-added products such as methanol or ethanol. Throughout the process, CO₂ will step through several intermediate states, each separated by an “energy barrier” that determines how fast or efficiently the reaction proceeds. For example, CO₂ can be transformed into formic acid or formaldehyde on its way of transformation into methanol.
By modelling these transitions in detail, scientists can pinpoint which steps go fast and which atomic configurations help the reaction move forward more effectively. It is not only important to predict the activity of transition metals in this catalytic process, but also their selectivity. For example, methane is a low-value product in CO₂ hydrogenation, while oxygenates such as methanol or acetic acid are high-value products. Thus, modeling of extended networks of possible transformations is essential to predict the outcomes of the catalytic process with various nanoparticle catalysts and design better catalysts for this reaction with the help of simulations.
Through this research, the team is uncovering how tiny metal catalysts anchored on 2D materials help activate hydrogen and move it across the catalyst surface, a fundamental step for driving CO₂ hydrogenation. With the support of advanced computer simulations, computational chemists and materials scientists collaborate closely to refine laboratory experiments, bridging the gap between theory and application and accelerating the design of next-generation catalysts.
This computational project was carried out in close collaboration with experimentalist teams at A*STAR, who translated mechanistic insights into real-world catalyst design. This collaboration resulted in a few patents and technology disclosures for novel methods of producing alcohols and fuels via CO₂ hydrogenation, and filed by A*STAR ISCE2.
Traditional catalysts tend to lose activity or selectivity over time, because nanoparticles clump together or degrade. The nanoparticle-on-2D approach changes that. By anchoring metal atoms on atom-thin, stable supports, researchers can:
- Prevent nanoparticle aggregation, or clumping, to maintain long-term catalytic performance
- Enhance hydrogen activation, to easily break CO₂’s tough carbon–oxygen bonds
- Map critical high-barrier steps, to offer insights for rational catalyst design
Beyond advancing practical catalyst design, the research also deepens our understanding of CO₂ hydrogenation and fundamental chemistry.
Modeling complex catalytic processes, such as CO₂ hydrogenation, is not an easy task. Usually, quantum chemical methods are used to model catalytic reactions. However, exhaustive quantum chemical modeling would be much too inefficient.
HPC resources provided by NSCC Singapore allowed the researchers to use quantum chemical methods to obtain high-accuracy data representing CO₂ hydrogenation on various metal nanoparticles, which served as a training dataset for their data-efficient machine learning (ML) model. In particular, the team employed a neural network that represents atomic structures of the reacting species and catalyst, enabling robust predictions across possible transformations of CO₂. Using the developed ML approach, the team was able to rapidly predict the energy barriers in CO₂ hydrogenation with high accuracy. In addition, the team automated the analysis of the transformations between intermediate states, which allowed them to gauge the properties of the hypothetical “optimal” catalyst.
The team upholds the standards of Open Science, publishing these results in a series of publicly available preprints, which are now considered for publication in peer-reviewed journals.
Understanding catalytic reactions at the atomic scale demands immense computational power and speed. Each simulation involves thousands of atoms, billions of interactions, and countless quantum-level calculations Some calculations may involve thousands or even millions of atoms and must be repeated under varying conditions, while others require countless accurate quantum-level computations that depend on cutting-edge processors operating in parallel. With NSCC Singapore’s ASPIRE 2A supercomputer, researchers can:
- Run large-scale simulations of hydrogen splitting, migration, and CO₂ bond breaking that mimic real world reaction conditions
- Apply parallelisation and workload balancing, distributing calculations across numerous CPU nodes and thousands of CPU cores to drastically reduce runtimes and optimise efficiency
- Leverage molecular dynamics and density functional theory (DFT) codes optimised for high-performance environments.
Performance tuning on NSCC Singapore’s clusters has reduced computation time significantly, enabling the team to test multiple catalyst configurations in a feasible timeframe — work that would otherwise take years. On the one hand, NSCC Singapore’s ASPIRE 2A provides 800 CPU nodes, so the researchers can request numerous calculations running independently to obtain a representative sampling of different catalysts or reaction conditions. On the other hand, available software, through efficient parallelization, can accelerate each calculation from roughly twofold up to several orders of magnitude, depending on the system being modeled or software used.
The research holds the potential to reshape the energy and chemical industries, where sustainable production of methanol and higher alcohols could transform fuel and feedstock supply chains. It also contributes to the global effort in carbon capture, utilisation, and storage (CCUS) — turning emissions into valuable resources.
By enabling cleaner fuel production from captured CO₂, the project supports reduced greenhouse gas emissions and long-term energy sustainability, delivering benefits that extend beyond industry to society at large through cleaner air and a smaller carbon footprint.
In the near term, insights from HPC simulations are refining catalyst design and strengthening collaboration between computational and experimental research teams and strengthening academic understanding. Over the longer term, this knowledge could pave the way for scalable CO₂-to-fuel technologies that fundamentally change how we produce energy, chemicals, and materials.
Ultimately, the project supports Singapore’s vision to lead in sustainable innovation — demonstrating how advanced computation and materials science can work together to accelerate the global transition to a low-carbon future.
“The development of catalytic materials for CO2 conversion technologies is very challenging due to the high complexity of the involved reaction mechanisms and employed material nanostructuring. NSCC resources allowed us to perform detailed simulations of the reaction network of CO2 hydrogenation on catalytic nanoparticles to guide the design of advanced nanoalloy catalysts for this reaction.”

Sergey Kozlov
National University of Singapore
Assistant Professor at the Department of Chemical and Biomolecular Engineering